Electron Dot Structure For Cbr4
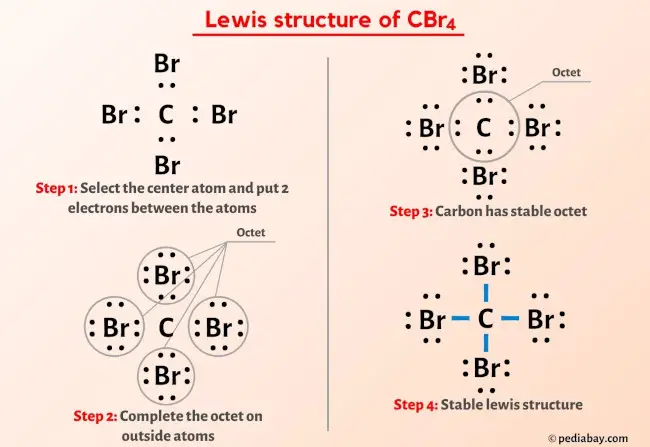
So you accept seen the above image past now, right?
Allow me explain the above epitome in short.
CBr4 lewis structure has a Carbon cantlet (C) at the center which is surrounded by four Bromine atoms (Br). There are four single bonds between the Carbon atom (C) and each Bromine atom (Br). There are 3 lone pairs on all the four Bromine atoms (Br).
If you oasis't understood anything from the above paradigm of CBr4 lewis construction, then but stick with me and y'all will go the detailed step by stride explanation on cartoon a lewis structure of CBr4.
So let'due south movement to the steps of drawing the lewis structure of CBr4.
Steps of cartoon CBr4 lewis structure
Pace 1: Find the total valence electrons in CBr4 molecule
In order to find the total valence electrons in a CBr4 molecule, starting time of all you lot should know the valence electrons present in carbon atom as well equally bromine atom.
(Valence electrons are the electrons that are present in the outermost orbit of any atom.)
Here, I'll tell you how you can easily observe the valence electrons of carbon besides every bit bromine using a periodic table.
Total valence electrons in CBr4 molecule
→ Valence electrons given by carbon atom:
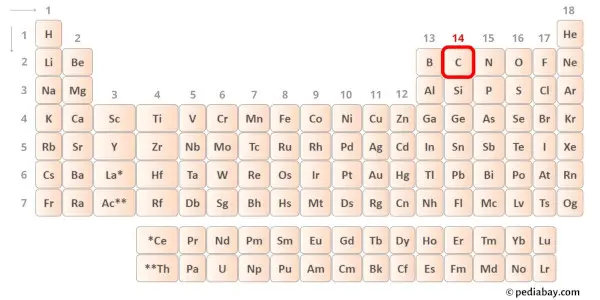
Carbon is group 14 chemical element on the periodic table. Hence the valence electrons present in carbon is 4.
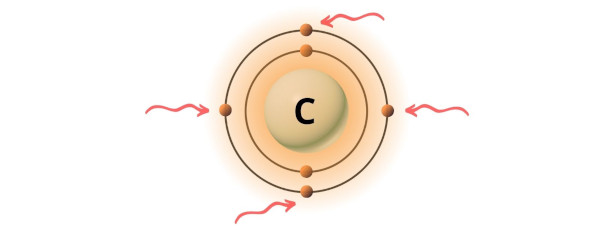
You can see the 4 valence electrons present in the carbon atom as shown in the above paradigm.
→ Valence electrons given by bromine cantlet:
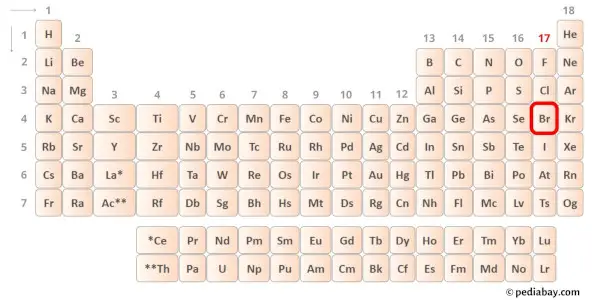
Bromine is a group 17 element on the periodic table. Hence the valence electrons nowadays in bromine is 7.
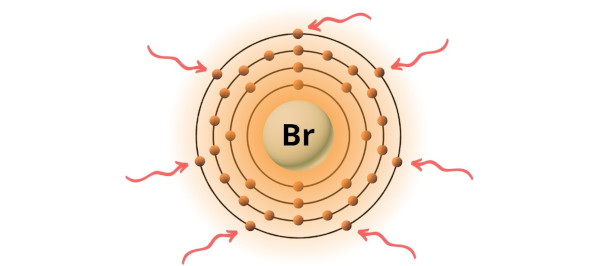
Y'all can see the 7 valence electrons present in the bromine atom equally shown in the above image.
Hence,
Total valence electrons in CBr4 molecule = valence electrons given by 1 carbon atom + valence electrons given past four bromine atoms = iv + 7(4) = 32.
Step 2: Select the primal cantlet
For selecting the heart atom, you lot take to remember that the atom which is less electronegative remains at the heart.
Now here the given molecule is CBr4 and it contains carbon atom (C) and bromine atoms (Br).
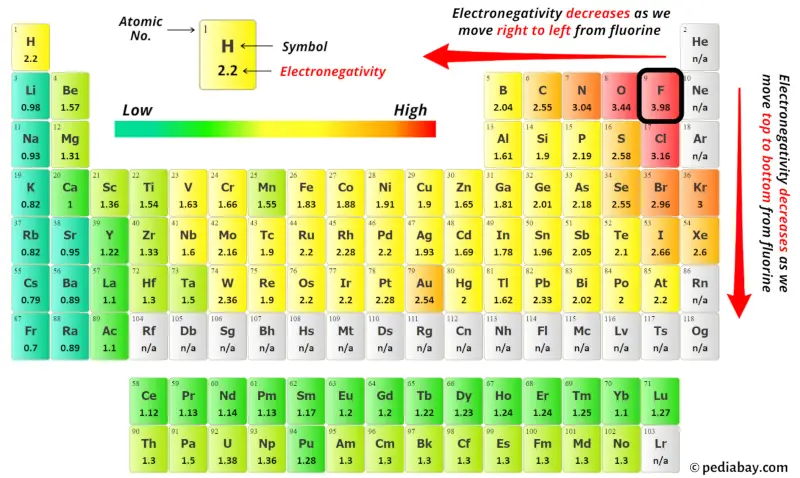
Yous can see the electronegativity values of carbon atom (C) and bromine cantlet (Br) in the above periodic table.
If we compare the electronegativity values of carbon (C) and bromine (Br) and then the carbon atom is less electronegative.
So here the carbon atom (C) is the middle atom and the bromine atoms (Br) are the outside atoms.
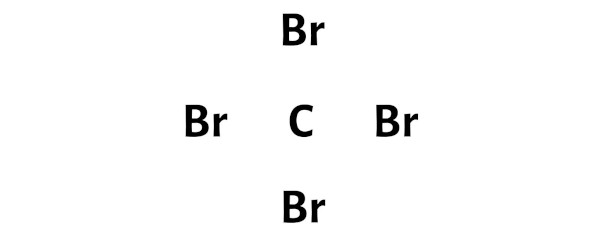
Step 3: Connect each atoms past putting an electron pair betwixt them
Now in the CBr4 molecule, y'all take to put the electron pairs betwixt the carbon atom (C) and bromine atoms (Br).
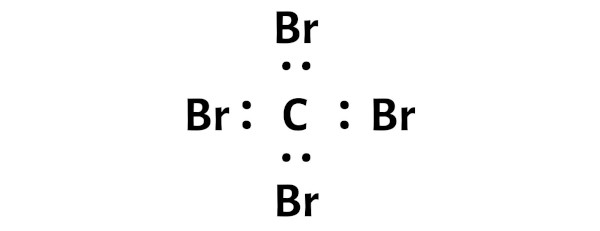
This indicates that the carbon (C) and bromine (Br) are chemically bonded with each other in a CBr4 molecule.
Stride 4: Make the outer atoms stable
At present in this footstep, you take to check the stability of the outer atoms.
Here in the sketch of CBr4 molecule, you tin run into that the outer atoms are bromine atoms.
These outer bromine atoms are forming an octet and hence they are stable.

Likewise, in footstep ane we accept calculated the full number of valence electrons present in the CBr4 molecule.
The CBr4 molecule has a full 32 valence electrons and all these valence electrons are used in the above sketch of CBr4.
Hence there are no remaining electron pairs to be kept on the central atom.
Then now let'south proceed to the side by side footstep.
Pace 5: Check the octet on the central cantlet
In this stride, yous have to check whether the central carbon atom (C) is stable or not.
In order to check the stability of the central carbon (C) atom, nosotros accept to check whether information technology is forming an octet or not.
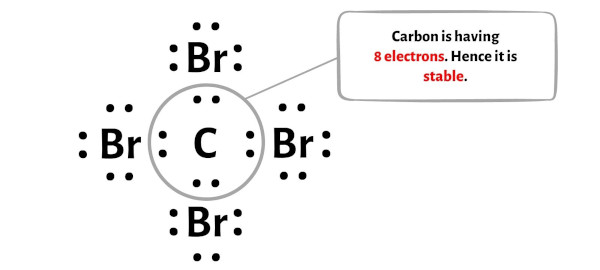
You tin can see from the to a higher place flick that the carbon cantlet is forming an octet. That means it has 8 electrons.
And hence the central carbon atom is stable.
At present let's proceed to the concluding step to check whether the lewis structure of CBr4 is stable or not.
Step 6: Check the stability of lewis structure
Now you have come to the terminal step in which you have to check the stability of lewis structure of CBr4.
The stability of lewis structure can be checked by using a concept of formal charge.
In short, at present you have to find the formal charge on carbon (C) atom besides as bromine (Br) atoms present in the CBr4 molecule.
For calculating the formal accuse, you take to use the following formula;
Formal charge = Valence electrons – (Bonding electrons)/ii – Nonbonding electrons
You tin can see the number of bonding electrons and nonbonding electrons for each atom of CBr4 molecule in the image given beneath.
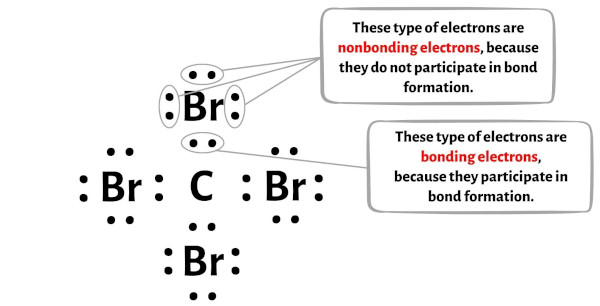
For Carbon (C) atom:
Valence electrons = 4 (because carbon is in grouping 14)
Bonding electrons = 8
Nonbonding electrons = 0
For Bromine (Br) atom:
Valence electron = 7 (because bromine is in group 17)
Bonding electrons = 2
Nonbonding electrons = 6
| Formal accuse | = | Valence electrons | – | (Bonding electrons)/ii | – | Nonbonding electrons | ||
| C | = | 4 | – | 8/two | – | 0 | = | 0 |
| Br | = | vii | – | 2/two | – | half dozen | = | 0 |
From the above calculations of formal accuse, you can see that the carbon (C) atom too equally bromine (Br) atom has a "zippo" formal charge.
This indicates that the above lewis construction of CBr4 is stable and at that place is no farther modify in the above construction of CBr4.
In the above lewis dot structure of CBr4, you can as well represent each bonding electron pair (:) as a single bond (|). Past doing then, you will get the following lewis structure of CBr4.
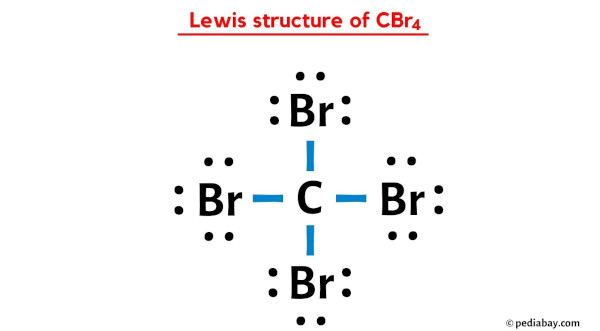
I hope you take completely understood all the above steps.
For more do and ameliorate agreement, you can try other lewis structures listed below.
Attempt (or at least See) these lewis structures for amend agreement:
Electron Dot Structure For Cbr4,
Source: https://pediabay.com/cbr4-lewis-structure/
Posted by: mcdonaldyone1997.blogspot.com


0 Response to "Electron Dot Structure For Cbr4"
Post a Comment